Authors :
Lotens¹, T. Najdovski¹, N. Valensart¹, N. Cellier¹, C. Sumian², Q. Brebant², C. Naegelen³, C. Cretenet³, N.Marpaux³
¹Blood Service, Belgian Red Cross, Namur, Belgium
²MacoPharma, Tourcoing, France
³EFS Bourgogne Franche Comté, Besançon, France
Background and Aim :
–> The plasticizer di(2-ethylhexyl)-phthalate (DEHP) is a common component in blood collection systems.
–> Exposure to DEHP is raising concern on its potential carcinogenicity and reprotoxicity. Therefore, the DEHP will be banned following the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation in 2025.
–>Macopharma is developing a non-DEHP whole blood collection system made of Dioctyl terephthalate (DEHT) plasticizer and phosphate-adenine-glucose-guanosin-saline-mannitol (PAGGSM) additive solution.
–>This solution has been tested both by the “Etablissement Français du Sang” (EFS) and by the “Service Francophone du Sang” (SFS) in a collaborative and multiparametric study.
AIM: Evaluate the solution proposed by Macopharma in DEHT/PAGGSM regarding the processing performances and the compliance of the red cell concentrates (RCC) and the plasma components to the European Directorate for the Quality of Medicines (EDQM), 20th version.
Study design and methods :
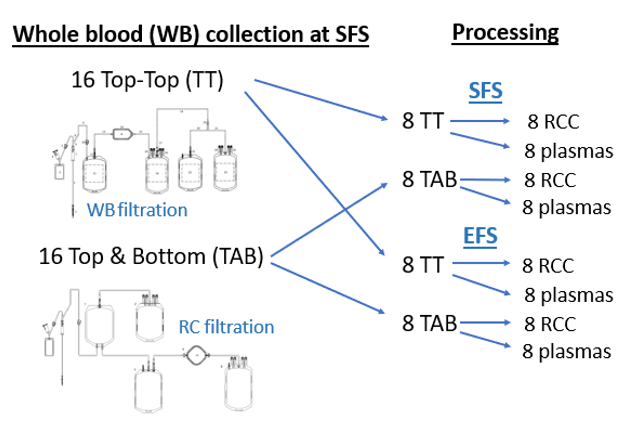
Results :
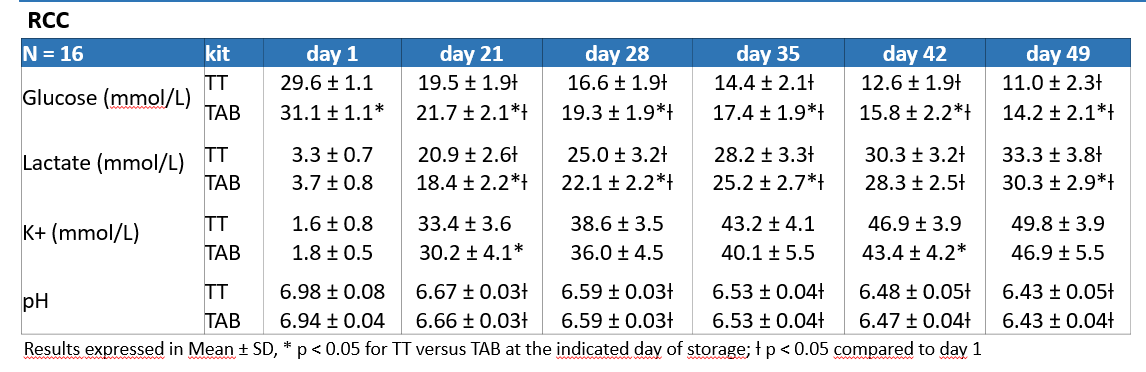
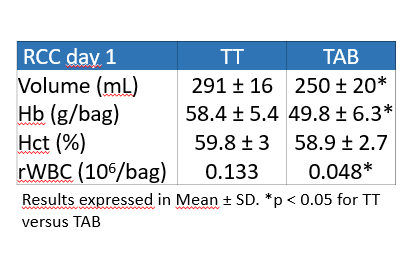
WB mean collected volume : 478±5 mL with 53% of the donations from group O (n= 32).
Hb and Hct are compliant to the EDQM until day 49.
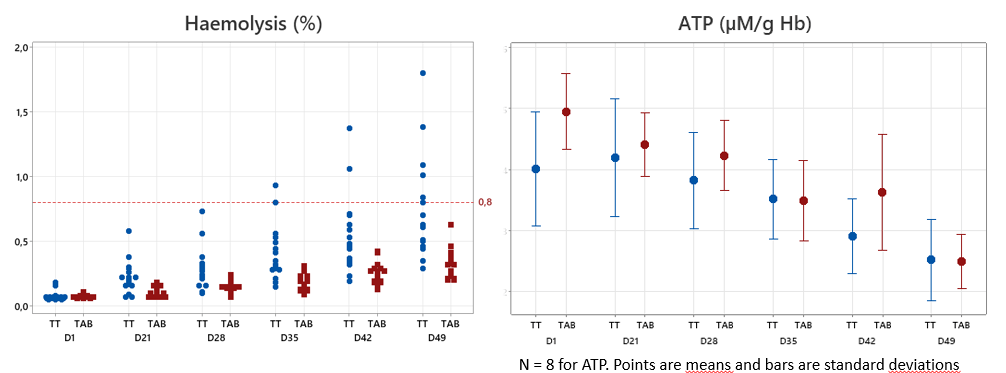
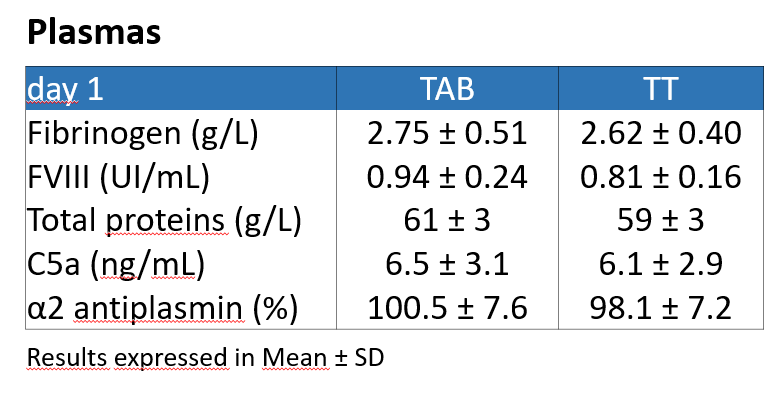
For all the plasma prepared, the rWBC results were below 0,1×109 cells/L and below 1×106 cells/unit for TAB and TT, respectively, below 50×109 residual platelets and below 6×109 residual red cells. 7 non-compliant results out of 32 were obtained with a FVIII below 0.70 IU/mL.
Conclusion :
–> DEHT-PAGGSM blood bag devices do not affect the quality results on the processing at day 1
–> For TAB bags, all the RCC units are compliant to the EDQM until day 49
–> For TT bags, all the RCC units are compliant to the EDQM until day 49, except for haemolysis which is 100% conform until day 28
–> All the plasma units are compliant to the EDQM except for FVIII with 80% of conformity. The FVIII is nevertheless compliant to the EFS local regulations. The non-conformities are likely attributed to the thermosensitivity of the FVIII and the late testing at 24 hours for the plasma.