Authors
Susanne Marschner*, Kathleen Kelly*, Crystal Stanley*, Dalia Moreno**, and Ludwig Frontier**
*Vitalant Innovation Center, Denver, CO
** Macopharma
Discover the poster in video : https://youtu.be/cWBCw0nbwYE
Background
As platelet (PLT) demand increases and the donor pool ages, US blood centers face mounting pressure to meet demand. Most US PLT concentrates are collected by apheresis, leaving whole blood (WB) underutilized. The Platelet Rich Plasma (PRP) method (top & top) is the only approved process to manufacture PLTs from WB held up to 8 hrs at room temperature (RT). Other countries use the buffy coat (BC) method (top & bottom) for the separation of WB into RBC, plasma, and a BC
layer after an overnight hold of WB. BCs are then pooled to produce leukoreduced PLTs in additive solution (PAS). Advantages of the BC method compared to the PRP method are higher platelet and plasma recovery and improved logistics due to the extended hold time of WB. Adopting this approach in the US could maximize the use of the existing WB donor pool, stabilize PLT inventory and mitigate PLT shortages. This is the first US study manufacturing leukoreduced PLT concentrates from pooled buffy coats using a novel DEHT plasticizer. The study was conducted at Vitalant, one of the largest US blood centers, in collaboration with Macopharma.
Methods
ABO-matched WB units were collected at satellite donation centers and transported to the blood center at RT. WB (500mL ± 10mL) was collected into 70mL citrate-phosphate-dextrose (Composelect, Fresenius Kabi) and held overnight at room temperature (RT). Within 24 hrs of collection, WB was transferred to a dry top/bottom bag set with DEHT plasticizer (Macopharma) and separated into RBCs, plasma, and individual BCs using an automatic press (Macopress Smarter) as outlined in Figures 1 and 2. RBCs were resuspended in additive solution (PAGGSM), leukoreduced and moved to 4˚C storage for 42 days. BCs rested for a minimum of 2 hrs at RT, and 5 ABO compatible BCs were pooled in PAS-E (SSP+) using a pooling set (Macopharma) and a multiple sterile connection device (Maconnect), that makes 6 sterile connections simultaneously. The BC pool was centrifuged, separated and leukoreduced using Macopress Smarter. Samples for bacterial culture were removed >48 hrs after collection of the “youngest” unit in the pool. PLT concentrates were stored for 7 days and sampled on days 1, 5, and 7 for in vitro quality. To manufacture components using the PRP method, collected WB was manually separated using a top & top disposable set. RBCs were leukoreduced and stored in AS-1 for 42 days. Five PRP units we pooled and leukoreduced (Imugard, Terumo), sampled for bacterial culture and stored for 7 days.
Processing Timelines
Figure 1: First spin separating RBCs and plasma. Timing representative of Standard processing in Europe.
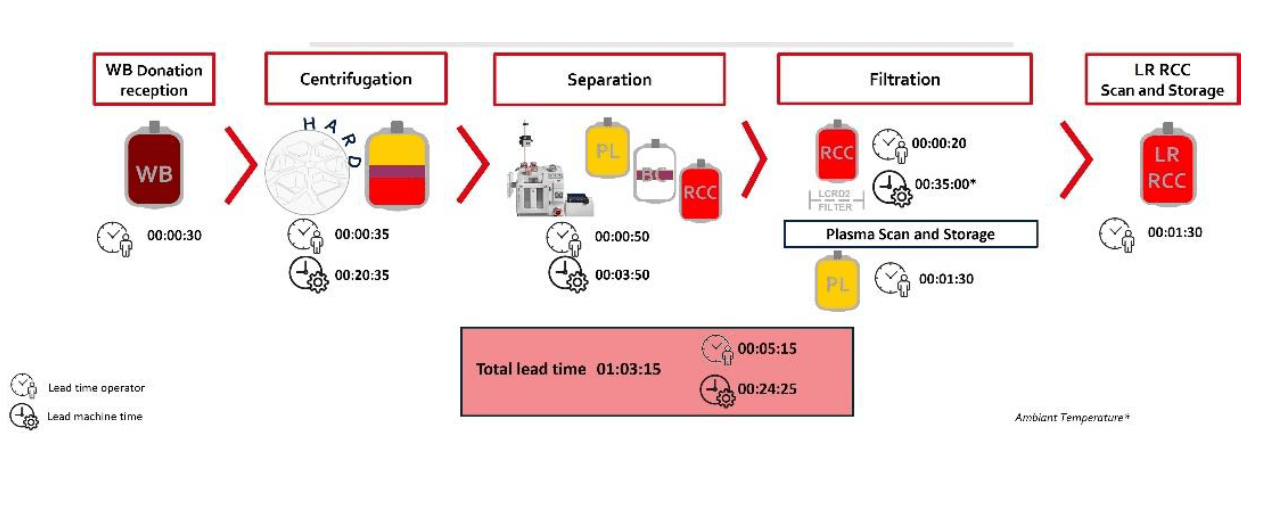
Figure 2: Second spin separating pooled platelets. Timing representative of Standard processing in Europe.
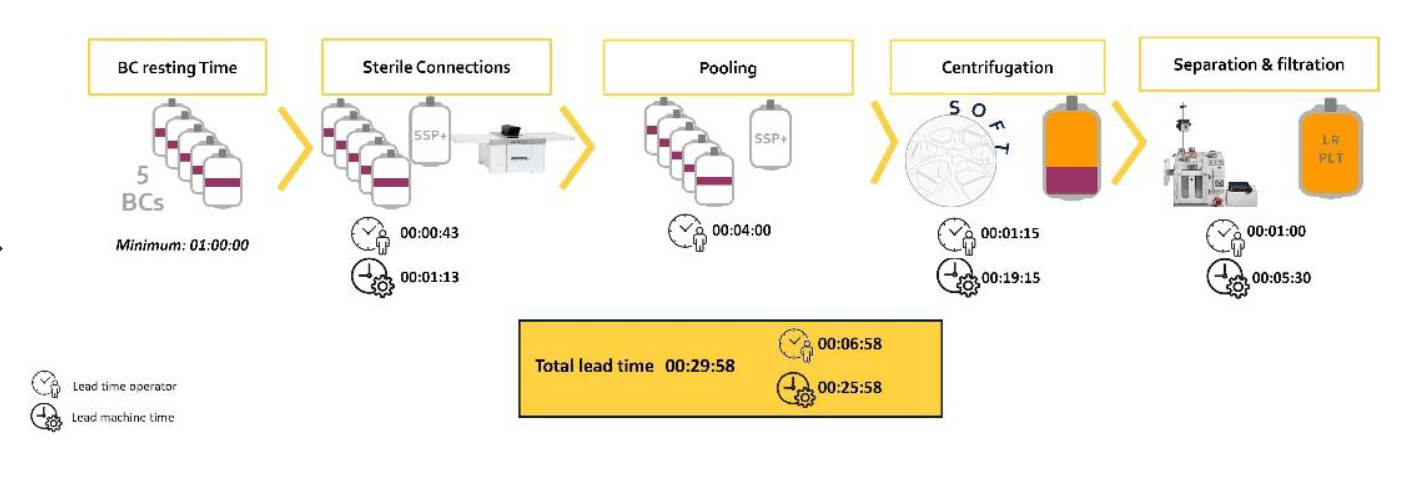
Table 1: In-vitro test results
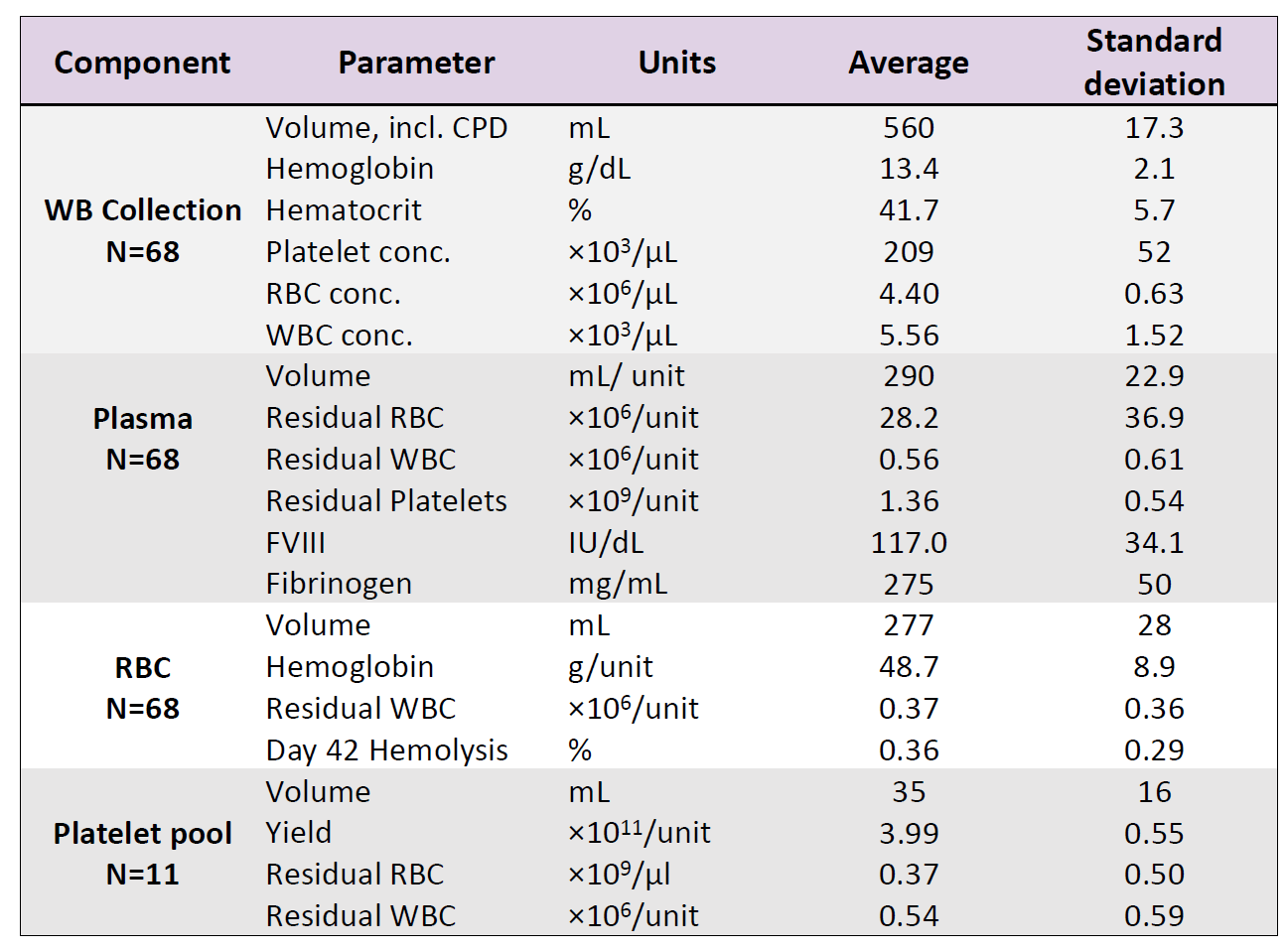
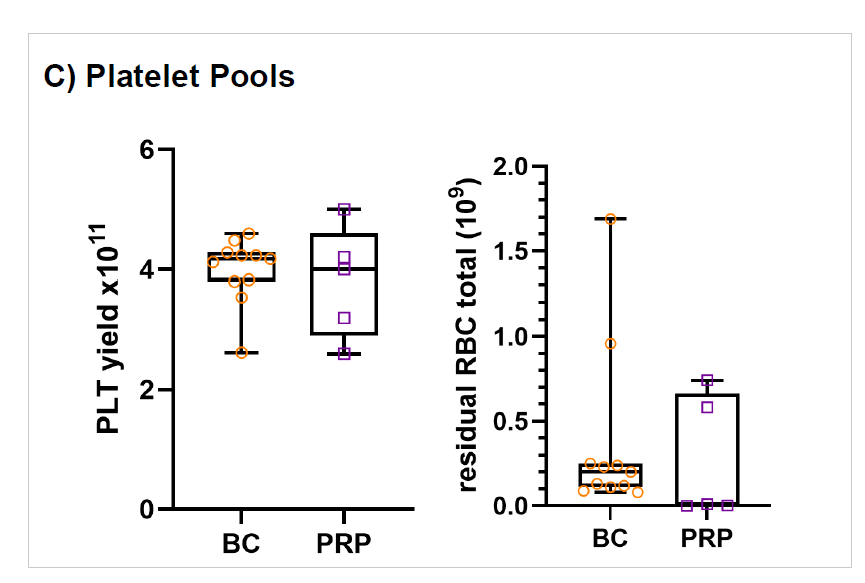
Figure 3: Platelet pools in-vitro storage parameters
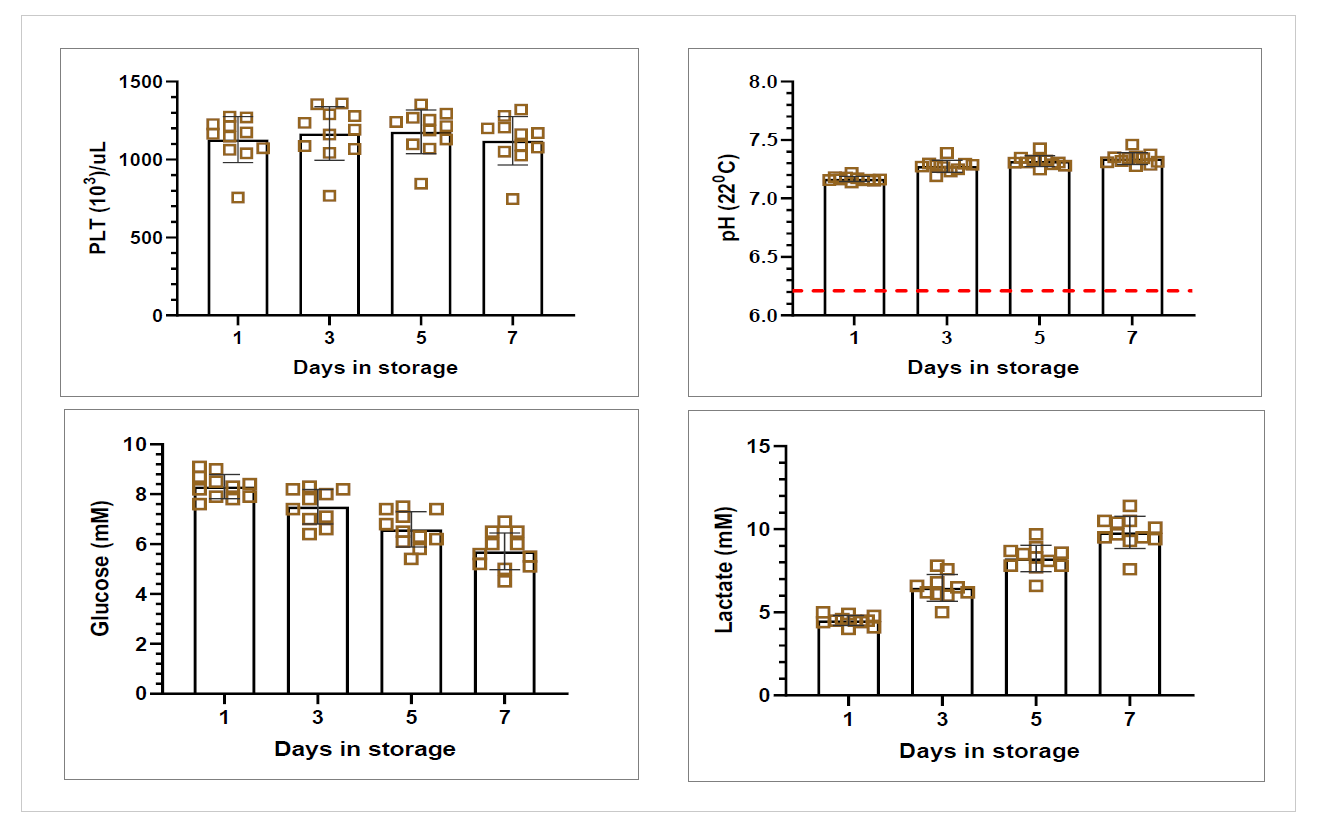
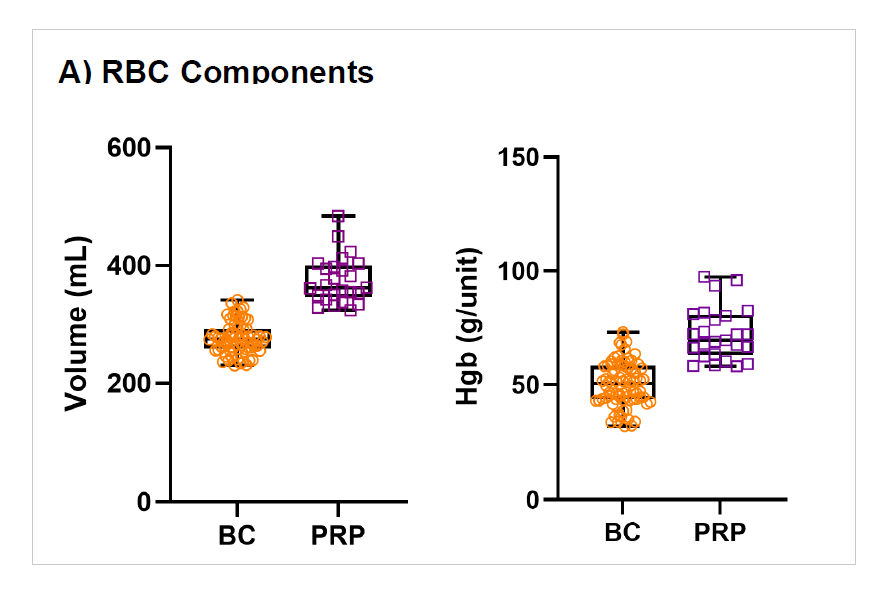
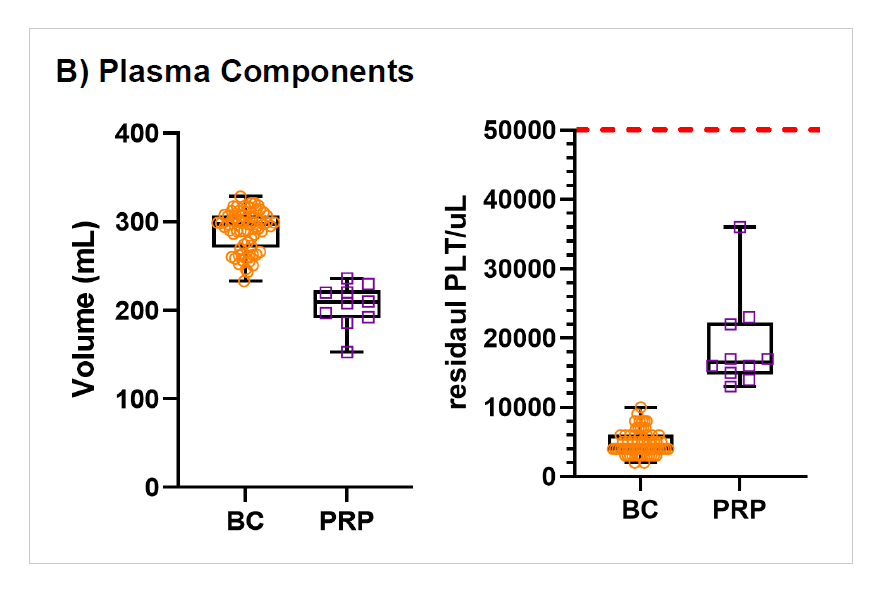
Figure 4: Comparison BC- and PRP-method
Results
WB (n=68) were collected and processing started on average within 19hrs (range 13.2-23.5 hrs) of collection and RBCs were moved to 4⁰C storage 21.4 ± 2.6 hrs post collection (range of 15.1-25.8 hrs. RBC recovery (vol/vol) was 97±1%. Plasmas were ready for -20⁰C storage after 20.5 ± 2.3 hrs (range 14.3-24.4 hours). Component characteristics and in vitro storage parameters are summarized in the Table 1. Swirling was observed throughout storage of all PLT pools. WB (n=25) separated using the PRP method were processed 4.75±2.5 hrs post collection. Plasma component data is from a subset of units (n=10) after implementation of optimized PRP separation steps. Key parameters of components separated by the BC- versus the PRPmethod are shown in Figure 4.
Conclusions
Processing after a 24 hrs hold at RT with the BC method in top & bottom DEHT plasticizer (non-DEHP) bags, coupled with automated separation and pooling technologies, demonstrates acceptable component quality and leukoreduction performance. Compared to the current manual PRP method, the RBC volume and Hgb content is decreased with the BC method whereas significantly more plasma is recovered. The BC method optimizes WB operations while maintaining high quality blood components, providing a sustainable solution to enhance PLT and plasma availability and resource efficiency.