Volume of collection : Up to 250 mL
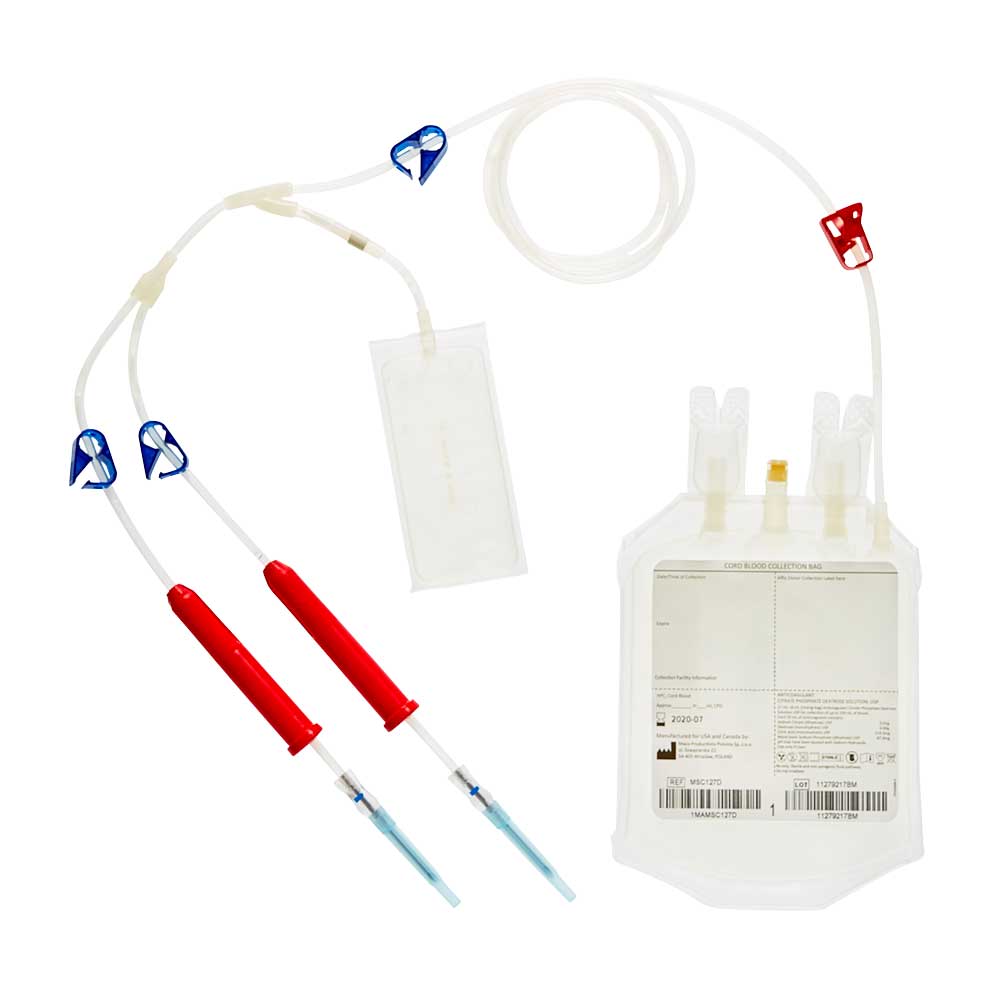
USA – FDA Approved collection bags
Our cord blood collection bags include a variety of innovative features. Macopharma provides two designs of bags developed specifically for cord blood collection. Graduations to provide an indication of collected volume, an additional 12G needle for the MSC127D reference but also an additional CPD rinsing pouch. For both references, the exterior of the bag is sterile inside an inner overwrap making the product suitable for caesarian section deliveries.
Our cord blood collection bags are FDA approved.
Our dual needle cord blood collection bag is FDA approved.
REF.MSC127D
Anticoagulant solution : 27 mL CPD* in the collection bag + 8 mL in the rinsing bag
Needle size : 12 G
Features : 2 needles + rinsing bag + Permanent red clamp
Shelf life: 2 years
Suitable for caesarean section deliveries. Sterile inside its inner overwrap
*Citrate Phosphate Dextrose, Manufactured exclusively for the U.S.A.
REF.MSC123D
Volume of collection : Up to 210 mL
Anticoagulant solution : 35 mL CPD* in the collection bag*
Needle size : 12 G
Features : 1 needles + Permanent red clamp
Shelf life: 2 years
Suitable for caesarean section deliveries. Sterile inside its inner overwrap
*Citrate Phosphate Dextrose, Manufactured exclusively for the U.S.A.
This information is for exclusive use of healthcare professionals.
The products are not available in every country, please contact your sales representative.
MACOPHARMA • Rue Lorthiois 59240 Mouvaux France • Tel : + 33 (0) 3 20 11 84 00 • Fax : +33 (0) 3 20 11 84 03
Société par actions simplifiée au Capital de 493.115 € RCS Lille Metropole 391 600 905 • Code APE 4641 Z • TVA FR 313 916 009 05